AXL Inhibition Restores Response to PD-1 Blockade in STK11/LKB1-mutant NSCLC
August 15, 2022
Clinical Trials at UT Southwestern
As an academic medical center, UT Southwestern offers clinical trials that give eligible patients access to the newest therapies. Learn more about clinical trials at UT Southwestern.
New research could benefit patients receiving treatment for lung adenocarcinomas.
Mutations in STK11/LKB1 occur in approximately 20% of patients with non-small cell lung cancer (NSCLC) and are associated with poor outcomes, specifically limited response to immune checkpoint blockade (ICB) therapy, which includes anti-programmed cell death protein 1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors. Due to multiple mechanisms of resistance, most of which are unknown, the benefit of ICB therapy alone is limited.
KRAS-mutant NSCLC tends to have a higher response rate to PD-1/PD-L1 inhibition compared with KRAS wild-type NSCLC; however, when KRAS is co-mutated with STK11/LKB1, responses to ICB are usually poor. Thus, strategies that sensitize STK11/LKB1-mutant NSCLC to ICB therapy would be beneficial to patients receiving treatment for lung adenocarcinomas. It’s one example of how Simmons Cancer Center is advancing cutting-edge, bench-to-bedside interventions.
New research published in Cell Reports Medicine by Rolf Brekken, Ph.D., a Professor in the Departments of Surgery and Pharmacology at UT Southwestern Medical Center and a member of the Simmons Cancer Center Experimental Therapeutics Research Program, suggests that an investigational drug targeting AXL, a receptor tyrosine kinase, could restore the ability of STK11/LKB1-mutant NSCLCs to respond to ICB therapy. He and his team hypothesize that AXL inhibition might be an effective strategy to potentially expand PD-1+ tumor-specific T cells and improve ICB efficacy.
“AXL targeting is being explored by multiple companies due to its association with tumor types prone to therapeutic resistance,” Dr. Brekken says. “Activation of AXL on innate immune cells suppresses type 1 interferon production, a critical signaling pathway responsible for the activation of anti-tumor CD8 T cells.”
Preclinical Study
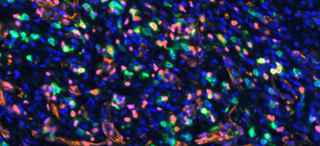
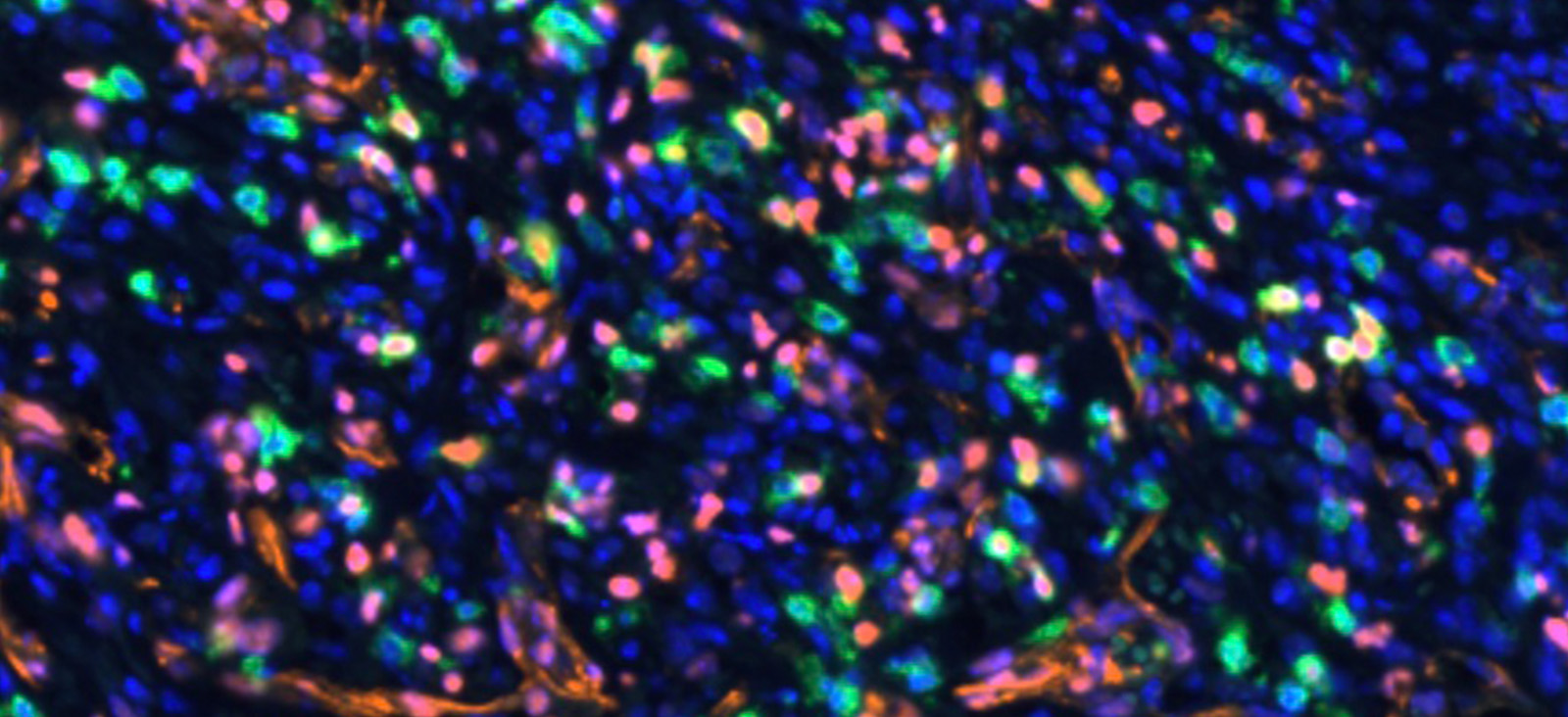
Dr. Brekken and his team introduced an STK11/LKB1 mutation into a preclinical mouse model of lung cancer, which resulted in ICB-refractory tumors. They postulated that the lack of response occurred due to the absence of a specific population of immune cells (TCF1-expressing CD8+ immune T cells) in STK11/LKB1-mutated tumors. These immune cells were also reduced in human NSCLC tumors harboring STK11/LKB1 mutations.
Interestingly, they observed that inhibition of AXL by the investigational drug bemcentinib led to more type 1 interferon secretion from AXL-expressing dendritic cells, resulting in expansion of the TCF1-expressing CD8+ immune T cell population, ultimately restoring therapeutic response to ICB therapy. This was observed in both syngeneic immunocompetent mouse models and in humanized mice bearing STK11/LKB1-mutant NSCLC human tumor models.
“Our data strongly supports clinical testing of AXL inhibition in combination with PD-1 checkpoint blockade in patients with STK11/LKB1-mutant NSCLC.”
Rolf Brekken, Ph.D.
To support the preclinical findings, Dr. Brekken noted that an ongoing phase 2 clinical trial evaluating bemcentinib showed promising results in NSCLC patients carrying the STK11/LKB1 mutation who were concomitantly treated with PD-1/PD-L1 inhibitors. The results from three patients, all of whom experienced objective clinical response to combination therapy, were reported in the publication.
“Our preclinical results provide mechanistic insight into the paucity of stem-like T cells in STK11/LKB1 mutant tumors and a molecular rationale for the poor response of these tumors to ICB therapy,” Dr. Brekken says.
Next Steps
While this investigation focused on NSCLC, it is possible that the beneficial effect of AXL inhibition on ICB therapy will extend beyond STK11/LKB1-mutant NSCLC.
The sample size was small, but the results of the three patients with NSCLC treated with bemcentinib and pembrolizumab provide encouraging evidence that inhibiting AXL could re-sensitize human STK11/LKB1-mutant NSCLC to ICB therapy, especially given that tumors from the patients did not harbor KRAS mutations and had minimal to no PD-L1 expression, making them unlikely candidates to respond to anti-PD-1 therapy alone.
“Our data strongly supports clinical testing of AXL inhibition in combination with PD-1 checkpoint blockade in patients with STK11/LKB1-mutant NSCLC and potentially in other tumors that are refractory to PD-1 blockade and that also exhibit deficits in TCF1+ CD8 T cells,” Dr. Brekken says.
Questions Remain
It remains unclear how specifically the STK11/LKB1 mutation results in a differential response to therapy compared with STK11/LKB1 wild-type patients. In addition, there is evidence that patients with STK11/LKB1-mutant NSCLC have shown response to single-agent PD-1 blockade.
“We anticipate more clinical evidence will emerge as further studies are performed,” Dr. Brekken says.
Other UT Southwestern researchers who contributed to this study include Huiyu Li, Zhida Liu, Longchao Liu, Hongyi Zhang, Chuanhui Han, Luc Girard, Hyunsil Park, Anli Zhang, Chunbo Dong, Jianfeng Ye, Michael Peyton, Xiaoguang Li, Kimberley Avila, Xuezhi Cao, Shuiqing Hu, Md Maksudul Alam, Esra Akbay, Bo Li, and Yang-Xin Fu.
This work was supported by a sponsored research agreement from BerGenBio ASA and grants from the National Institutes of Health (R01 CA243577, U54 CA210181, and P30 CA142543), the National Institutes of Health SPORE (P50 CA070907 and U54 CA224065), the Cancer Prevention and Research Institute of Texas (CPRIT: RP160652, RP150072, and RP180725), the Effie Marie Cain Foundation, and a CPRIT training grant (RP210041). Drs. Huiyu Li, Zhida Liu, John Minna, and Brekken are authors of a patent related to this study. Other researchers’ financial interests are disclosed in the study manuscript.
Rolf Brekken, Ph.D., is a Professor in the Departments of Surgery and Pharmacology at UT Southwestern Medical Center. He also serves as Deputy Director of the Hamon Center for Therapeutic Oncology Research and Chair of the Cancer Biology Graduate Program.