Cellular Stress Response Promotes Immune Evasion in Lung Cancer
October 26, 2020
Clinical Trials at UT Southwestern
As an academic medical center, UT Southwestern offers clinical trials that give eligible patients access to the newest therapies. Learn more about clinical trials at UT Southwestern.
Researchers uncover a key mechanism that regulates PD-L1, a protein that helps tumors evade the immune system and is the target of several immunotherapy drugs.
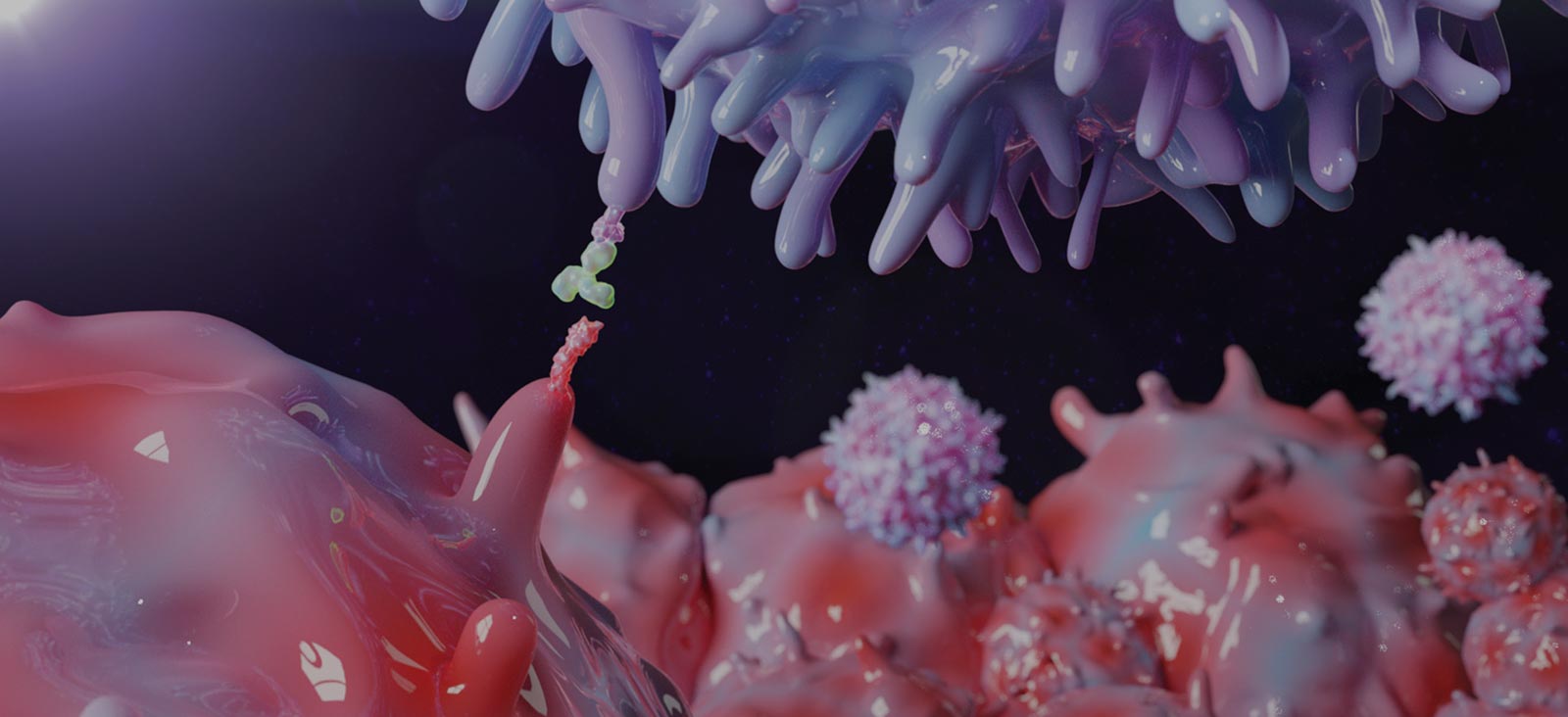
Lung cancer is the leading cause of cancer-associated deaths worldwide, with limited effective treatments and frequent recurrence. [1] Lung tumor cells frequently express high levels of programmed death-ligand 1 (PD-L1), a ligand of the PD-1 receptor on T cells, allowing tumors to directly suppress the host immune response by inhibiting T-cell proliferation and function. This ligand-receptor interaction is referred to as an immune checkpoint, and understanding how tumor cells exploit it to escape antitumor immunity is critical to developing novel treatment approaches. [2]
Clinical trials utilizing monoclonal antibodies that disrupt this PD-1/PD-L1 immune checkpoint have yielded remarkable results, with PD-1 immunotherapy approved as a first-line therapy for lung cancer patients. [3,4] Despite significant progress in targeting this pathway, the mechanisms through which PD-L1 is upregulated in non-small cell lung cancer and other tumor types is not completely understood.
This led us to perform a genome-wide screen to search for new regulators of PD-L1. [5] We reasoned that the identification of new PD-L1 regulators might allow us to uncover clinically relevant biomarkers that predict response and resistance to immunotherapy, which might lead to new therapeutic strategies to trigger antitumor immune responses and improve clinical outcomes.
Identifying Immune Checkpoint Regulators
To screen for new PD-L1 regulators, we leveraged the powerful gene-editing system, CRISPR-Cas9, that is being widely used to knock out genes of interest. We infected human lung cancer cells with a genome-wide lentiviral CRISPR library targeting more than 19,000 genes. Cells with the highest and lowest surface PD-L1 expression were collected by fluorescence-activated cell sorting with an antibody that recognizes endogenous cell surface PD-L1. This screening system proved to be highly effective, identifying known regulators of PD-L1 and revealing new factors that are critical for regulation of this immune checkpoint protein.
Using this unbiased functional screening approach, we found that a potent inhibitor of PD-L1 is a gene called uroporphyrinogen decarboxylase (UROD), which plays a key role in heme production. This iron-containing compound is crucial for carrying oxygen in red blood cells but is also important for viability of many other cell types. Our study revealed potent induction of PD-L1 upon disruption of heme biosynthesis.
Impairment of heme production activates the integrated stress response (ISR), a pathway that cells use in response to diverse stress conditions, including low oxygen or nutrient starvation, to maintain homeostasis. This allowed for enhanced PD-L1 production and suppression of antitumor immunity. Intriguingly, we found that the ISR pathway resulted in increased PD-L1 expression at the level of protein translation.
We further demonstrated that PD-L1 translation in response to cellular stress requires the translation initiation factor eIF5B. Interestingly, eIF5B was previously shown to be a key driver of translation under hypoxic conditions or viral challenge, suggesting additional contexts that may engage this mechanism to activate the immune checkpoint in cancer cells. Even more surprising, we found that eIF5B is sufficient to induce PD-L1 even in the absence of ISR activation.
This finding was not only mechanistically informative for the cancer research community but is also clinically relevant for lung cancer patients. When we examined human clinical datasets, we found that the eIF5B gene is overexpressed or amplified in ~20% of lung adenocarcinoma patients and that high expression of eIF5B correlates with poor overall survival of these patients.
“This work highlights the importance of future studies to examine the complete translational program orchestrated by eIF5B in cancer and to evaluate its potential as a therapeutic target.”
Kathryn A. O’Donnell, Ph.D.
Looking Forward
Collectively, these findings revealed an unanticipated mechanism of immune checkpoint activation in lung cancer with important therapeutic implications. This work highlights the importance of future studies to examine the complete translational program orchestrated by eIF5B in cancer and to evaluate its potential as a therapeutic target. We also want to know whether the expression of eIF5B in tumors would allow us to predict how patients respond to PD-1/PD-L1 immunotherapy.
Finally, investigation of a relationship between ISR activation and immune evasion in cancer will be an important priority for our future work. Our data suggest that ISR pathway inhibition might induce antitumor immunity alone or in combination with existing immunotherapies to improve clinical outcomes. Indeed, a drug called ISRIB (short for “integrated stress response inhibitor”) that targets the ISR pathway has demonstrated efficacy in preclinical studies of aggressive prostate cancer.6 In future studies, it could be repurposed to be used in combination with immune checkpoint therapies.
About the Author
Kathryn O’Donnell, Ph.D., is an Associate Professor of Molecular Biology at UT Southwestern whose laboratory is focused on understanding the mechanisms that contribute to tumor initiation, progression, and metastasis. Her lab has made significant contributions to the development and utilization of in vivo and ex vivo transposon mutagenesis systems. They have identified novel genes that promote liver cancer, leukemia, and non-small cell lung cancer.
Footnotes
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424. doi:10.3322/caac.21492
2. Ribas A. Adaptive Immune resistance: How cancer protects from immune attack. Cancer Discov. 2015;5(9):915-919. doi:10.1158/2159-8290.CD-15-0563
3. Herbst RS, Baas P, Kim D-W, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small cell lung cancer (KEYNOTE-010): A randomised controlled trial. Lancet (London, England). 2016;387(10027):1540-1550. doi:10.1016/S0140-6736(15)01281-7
4. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1–positive non–small cell lung cancer. N Engl J Med. 2016;375(19):1823-1833. doi:10.1056/NEJMoa1606774
5. Suresh S, Chen B, Zhu J, et al. eIF5B drives integrated stress response-dependent translation of PD-L1 in lung cancer. Nat Cancer. 2020;1(5):533-545. doi:10.1038/s43018-020-0056-0
6. Nguyen HG, Conn CS, Kye Y, et al. Development of a stress response therapy targeting aggressive prostate cancer. Sci Transl Med. 2018;10(439). doi:10.1126/scitranslmed.aar2036