What Is Transcranial Magnetic Stimulation (TMS)?
Transcranial magnetic stimulation (TMS) is a painless and virtually side effect-free treatment for people with depression who have not responded to conventional pharmacological treatments. It was approved by the FDA for routine clinical use for depression in 2008.
TMS works by sending magnetic pulses into a targeted area of the brain involved with mood regulation. After a series of treatments, the magnetic pulses change how nerve cells function, helping to improve or, in many cases, resolve symptoms of depression.
The field of TMS research has evolved tremendously in the last decade. In 2018, a new TMS procedure, called theta-burst stimulation (TBS), was introduced. TBS delivers magnetic pulses in short bursts patterned after natural brain rhythms and produces similar results to standard TMS in a much shorter session.
More recently, accelerated TBS protocols have been developed, offering several short sessions per day for 1 to 3 weeks instead of a single daily treatment. These accelerated schedules are equally effective as standard protocols but shorten the overall treatment course. This reduces the number of clinic visits required.
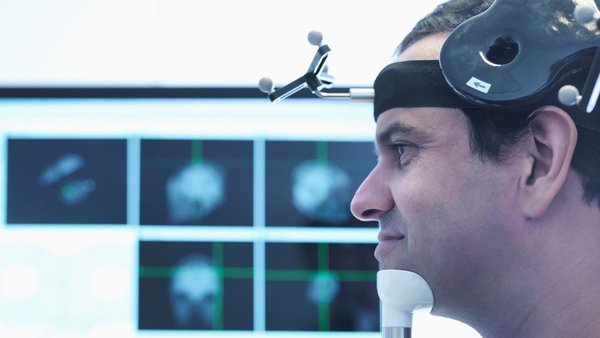
In addition, some patients may benefit from neuronavigation, a technique that works like a GPS for the brain. It uses the patient’s MRI scan to guide the magnetic coil to the exact spot in their brain that needs stimulation, improving accuracy. Most patients don’t need neuronavigation, but it can be useful in certain cases.