Novel Biomarker-Guided Therapeutics Target Glioma Origins
April 5, 2023
Clinical Trials at UT Southwestern
As an academic medical center, UT Southwestern offers clinical trials that give eligible patients access to the newest therapies. Learn more about clinical trials at UT Southwestern.
Expanding their suite of preclinical disease models, Simmons Cancer Center researchers identify a new targetable vulnerability in IDH-mutant gliomas.
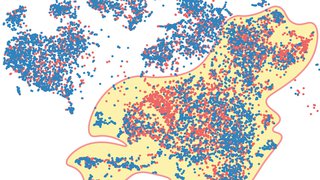
Neuro-oncology researchers at UT Southwestern Harold C. Simmons Comprehensive Cancer Center have pioneered a new patient-derived organoid model of lower-grade gliomas capable of identifying biomarker-guided therapeutic strategies poised for clinical translation. The model could potentially provide benefit above that of conventional experimental platforms.
“We developed an efficient method for producing faithful in vitro models of lower-grade gliomas,” says Samuel McBrayer, Ph.D., Assistant Professor at the Children’s Medical Center Research Institute at UT Southwestern and in the Department of Pediatrics. “Historically, developing patient-derived models of lower-grade gliomas has been challenging, contributing to few experimental platforms that support laboratory-based investigations of this disease.”
Conventional Models
Despite marked efforts to develop novel therapeutics, clinical progress for adult patients with gliomas has been limited. As a result, there remains a strong need for better glioma therapies. In contrast to the success rates associated with production of high-grade glioma cell lines from glioblastoma tissue samples, in vitro models of lower-grade gliomas have proved much more difficult to generate.
“Our understanding of the molecular pathogenesis of gliomas has grown considerably over the past two decades, driven in part by high-throughput sequencing,” says Toral Patel, M.D., Associate Professor of Neurological Surgery. “However, few in vitro models exist to support basic and translational research aimed at combating low-grade gliomas.”
“We hope our new model leads to more investigation, both preclinically and clinically, because the unmet medical need for glioma patients remains high”
Samuel McBrayer, Ph.D.

The New Model
Drawing on past glioma modeling work, the researchers hypothesized that by reducing oxygen tension to 5% (a level reported to be physiologically relevant for brain tissues), they could generate lower-grade glioma organoid models from patient tissue specimens.
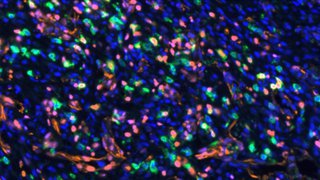
In a recent study, Dr. McBrayer and his team collected a series of 22 tissue specimens from glioma resection surgeries, including 15 lower-grade glioma samples, to directly test this hypothesis. The results showed that explanted lower-grade glioma organoids are viable and maintain parental tumor characteristics, including cytoarchitecture, cellular heterogeneity, proliferative capacity, and distinctive genomic alterations.
“Importantly, the organoids maintained molecular and histological characteristics of the primary tumors from which they were created,” Dr. McBrayer says. “Our findings outline a novel approach to produce in vitro models of lower-grade gliomas, directly addressing a long-standing challenge in the field.”
Identifying Actionable Targets
In a follow-up study, the researchers used the new organoid models to evaluate findings from a chemical synthetic lethality drug screen that revealed that IDH1-mutant glioma cells are hypersensitive to drugs targeting enzymes in the de novo pyrimidine nucleotide synthesis pathway, including dihydroorotate dehydrogenase (DHODH).
“IDH1 and IDH2 mutation testing is now routinely performed for all gliomas,” Dr. Patel explains. “We wanted to uncover metabolic vulnerabilities in IDH mutant gliomas that could yield new therapeutic targets.”
After developing a genetically engineered mouse model of mutant IDH1-driven astrocytoma, the researchers used it and multiple patient-derived models, including organoids, to show that the brain-penetrant DHODH inhibitor BAY 2402234 displays efficacy against IDH-mutant gliomas.
“Our work provides preclinical rationale to initiate clinical studies of BAY 2402234 in gliomas by identifying IDH mutational status as a predictive biomarker of response,” Dr. McBrayer says. “This new therapeutic strategy shows promise for treating IDH1-mutant gliomas that display de novo resistance to mutant IDH1 inhibitors.”
Clinical Trials
Based on the encouraging preclinical results, the team is planning to initiate an early-phase clinical trial for BAY 2402234 in 2023. Within the trial, correlative studies are also planned to better understand the effects of DHODH inhibition on immune cell populations in the microenvironment.
“We hope our new model leads to more investigation, both preclinically and clinically, as the unmet medical need for glioma patients remains high,” Dr. McBrayer says.
Samuel McBrayer, Ph.D., is an Assistant Professor at CRI and in the Department of Pediatrics at UT Southwestern and a Cancer Prevention and Research Institute of Texas (CPRIT) Scholar. His lab is working to identify the metabolic mechanisms that cause cells to become cancerous and find new ways to inhibit them. Dr. McBrayer is a member of the Cellular Networks in Cancer Research Program at Simmons Cancer Center.
Toral Patel, M.D., is an Associate Professor in the Department of Neurological Surgery at UT Southwestern. She is the Director of the UT Southwestern Brain Tumor Program and a member of UT Southwestern’s Peter O’Donnell Jr. Brain Institute and Simmons Cancer Center, where she is part of the Experimental Therapeutics Research Program. Dr. Patel specializes in neurosurgical oncology, focusing her research on improving drug delivery to brain tumors.