Beyond weight loss: GLP-1 RAs reduce heart attack, stroke risk for Type 2 diabetes patients
April 17, 2023

GLP-1 receptor agonists are having a moment.
Better known to the public by brand names such as Ozempic, Wegovy, Trulicity, Rybelsus, Victoza, Saxenda, and Mounjaro, these glucose-lowering medications, which were initially approved for the treatment of patients with type 2 diabetes, have made headlines recently thanks to their demonstrated effectiveness at treating obesity and helping people with or without diabetes lose weight.
Whereas much of the current attention surrounding GLP-1 RAs, or glucagon-like peptide-1 receptor agonists, has been focused on their unexpected and potent effect on weight loss, one important aspect of this class of drugs should not be overlooked:
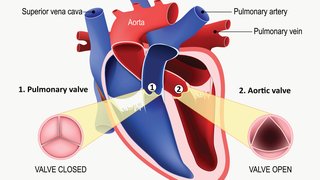
GLP-1 RAs have been shown to reduce the risk of heart attack, stroke, and other major adverse cardiovascular events (MACE) in patients with type 2 diabetes who have or are at high risk for atherosclerosis.

The discovery grew out of a series of eight large-scale, randomized clinical trials conducted around the world that tested these glucose-lowering drugs for heart safety.
Darren McGuire, M.D., M.H.Sc., a Professor in UT Southwestern’s Department of Internal Medicine and member of the Division of Cardiology, has played leadership roles in many of the trials over the last 15 years that have tested the cardiovascular safety of drugs used to treat type 2 diabetes. He is uniquely qualified to explain how GLP-1 RAs are changing the landscape for patients – with and without diabetes – and their providers.
What are the primary benefits of GLP-1 RAs?
This class of drugs has numerous metabolic effects, including increasing insulin secretion in response to high blood glucose. Initially, they were approved for the treatment of high blood glucose in patients with type 2 diabetes. Several GLP-1 RAs were subsequently shown to reduce cardiovascular risk in patients with type 2 diabetes with or at high risk for atherosclerosis. Three GLP-1 RAs (Victoza, Ozempic, and Trulicity) have been approved in the U.S. for the treatment of such patients independent of need for better blood glucose control.
When given on top of the best treatments for type 2 diabetes, blood pressure, and cholesterol, GLP-1 RAs reduce hemoglobin A1c, blood pressure, total cholesterol, and weight. But more important than modifying risk factors, the GLP-1 RAs provide patients with type 2 diabetes on average a 14% relative risk reduction for major cardiovascular events, including:
- Cardiovascular death
- Heart attack
- Stroke
Three GLP-1 RAs have been indicated by the FDA to reduce heart disease risk in people with type 2 diabetes who have or are at high risk for blockages of arteries of the heart, brain, and legs, also known as atherosclerotic cardiovascular disease (ASCVD):
- Dulaglutide, which is branded as Trulicity and is currently the only GLP-1 RA approved for cardiovascular disease risk reduction in patients with or without established ASCVD
- Liraglutide, which is branded as Victoza and was the first GLP-1 RA to show efficacy for heart benefits in a clinical trial
- Semaglutide, which has been increasingly prescribed off-label as a weight-loss medication under the brand name Ozempic
Whereas we are still exploring the biological mechanisms of how GLP-1 Ras benefit the heart, the body of evidence we have at present is already revolutionizing the care of patients with type 2 diabetes. Professional society guidelines and clinical care recommendations (for example, The American Diabetes Association Standards of Care in Diabetes-2023) are now recommending their use for all patients with type 2 diabetes who are at high cardiovascular risk.
An unexpected effect observed across the class of GLP-1 RAs in almost every study completed to date is their potent effect on weight loss. Based on these observations, the GLP-1 RAs have exploded in popularity for treating obesity and helping people lose weight, with two medications approved in the U.S. for the treatment of obesity, independent of diabetes status (Saxenda, a high-dose formulation of liraglutide) and Wegovy (high-dose semaglutide); and one presently under FDA review for the same (tirzepatide or Mounjaro).
How do GLP-1 RAs reduce cardiovascular risks?
GLP-1 RAs treat high blood glucose by increasing glucose secretion from the pancreas in response to glucose levels in the blood. Originally developed to treat high blood glucose for persons with type 2 diabetes, these medications also have been demonstrated to induce weight loss, reduce inflammation, increase satiety, and lower blood pressure.
Trial results indicate that the GLP-1 RAs appear to slow the progression of atherosclerosis (plaque) in the arteries and stabilize it, making it less likely to clog the arteries and less likely to become unstable to cause heart attacks and strokes.
Another class of medications initially developed for the treatment of high blood glucose in patients with type 2 diabetes, sodium-glucose cotransporter 2 inhibitors (SGLT2i), has also been demonstrated to reduce cardiovascular risk, with corresponding FDA product label changes and professional society recommendations endorsing their use.
Important differences exist regarding the cardiovascular effects of the GLP-1 RAs and SGLT2i medications, and these effects seem complementary. The GLP-1 RAs appear to modify atherosclerosis in the arteries, much like statin medications that treat cholesterol, and takes about a year or more to demonstrate measurable benefits on risk for cardiovascular events, whereas the SGLT2i medications seem to affect heart and kidney function more prominently and faster, with benefits evident very soon after starting the medication.
Related reading: The connection between diabetes and heart disease
What are the risks and side effects to consider?
All medical therapies carry some risk of side effects, and the initiation of GLP-1 RAs requires careful attention to set expectations.
Almost all patients will have some gastrointestinal side effects during the first week or two of taking the medication, but those symptoms typically resolve over the first two to four weeks and are rarely severe enough to stop therapy. Starting at too high of a dose or moving to the next higher dose too quickly can exacerbate GI symptoms such as indigestion, nausea, vomiting, diarrhea, or constipation to the point the patient may quit the therapy. There is no reason to start at anything but the lowest dose; and never an urgency to titrate up, so start low and go slow.
Before starting on a GLP-1 RA, patients and cardiologists should discuss several potential risk areas:
- Pancreatitis: There is an extremely low risk of developing pancreatitis with these medications, affecting approximately 1 in 10,000 patients. Pancreatitis can cause severe abdominal pain, nausea, and vomiting and may require hospitalization.
- Diabetic retinopathy: This phenomenon occurs when mild or previously undiagnosed diabetic retinopathy – an eye disease caused by chronic uncontrolled diabetes – suddenly gets worse after starting a GLP-1 RA. Patients should get evaluated for diabetic retinopathy with a retinal exam, preferably done within the 12 months before starting the medication.
- Advanced systolic heart failure: In patients with advanced systolic heart failure, the safety of GLP-1 RA remains uncertain. Results of two randomized placebo-controlled trials of liraglutide in patients with advanced systolic heart failure, with or without diabetes, failed to demonstrate benefit of liraglutide, and the trend in both trials was worse outcomes with liraglutide versus placebo. So, uncertainty remains about safety in this population and caution is advised, noting that this concern really only applies to those with the most severe heart failure.
How come GLP-1 RAs are not prescribed more by cardiologists?
Despite the robust body of evidence showing the cardiovascular benefits of GLP-1 RAs, their origins as “diabetes/glucose medications” and the mode of administration of all but one by subcutaneous injection have posed barriers for cardiologists to adopt them as a routine part of clinical practice. (Rybelsus is the only one taken as a pill; the others are injectables.)
Cardiologists and their clinic staff aren’t accustomed to teaching patients on a broad scale how to use injectable medications, since most heart drugs come in pill form. In addition, many cardiologists think prescribing GLP-1 RAs should be the domain of endocrinologists, even though there is an unequivocal body of evidence demonstrating cardiovascular efficacy, and recommendations for prescription are now in every guideline and professional society recommendation for cardiology, which parallel the recommendations from endocrinology and primary care societies.
Hesitancy among clinicians about prescribing these injectables and patients taking them should really not be an obstacle. The medications are all delivered using an easy-to-use pen injector with a needle so small that not only is it painless, patients barely feel it enter the skin. Also, there is no need for skin preparation with alcohol swabbing or other methods; just dial up the dose on the pen, bare the skin and inject. The entire process takes less than 30 seconds, and in my experience, a once-weekly injection (Ozempic, Trulicity, and Mounjaro) is preferred by many over a daily pill. As a cardiologist and someone with type 2 diabetes, I can attest that a weekly GLP-1 RA is easy to take and administer. I encourage every cardiologist to inject themselves with a practice device at least once to see how easy it is.
How much do GLP-1 RAs cost, and does insurance cover them?
GLP-1 RAs for the treatment of type 2 diabetes cost about $1,000 a month, and for obesity formulations, about $1,500 per month. While that may be cost-prohibitive, pressure is mounting for more health care insurers to cover GLP-1 RAs, especially for the treatment of type 2 diabetes, given the evidence of their heart benefits.
Our clinical pharmacist partners in the UT Southwestern and Parkland health care systems collaborate with us to help patients get a start on their GLP-1 RA medications. With a simple referral to a clinical pharmacist, they:
- Discuss insurance coverage and copay information with patients, and work to find the best medication at lowest out-of-pocket cost for each patient.
- Counsel patients on what side effects to expect and how to manage them.
- Teach patients to give themselves injections.
- Monitor the initiation and guide the patient through titrating from lowest dose to the target dose of medication.
This partnership with our clinical pharmacists has made it easier for cardiologists to prescribe GLP-1 RA medications and get them to the patients who will benefit from them most.