Impact of COVID-19 on Cancer Clinical Research at Simmons Cancer Center
January 30, 2022

It was quite a year for change and discovery.
In response to the COVID-19 pandemic, government agencies, including the U.S. Food and Drug Administration (FDA) and the National Institutes of Health (NIH), suggested major changes to simplify the conduct of clinical trials and to limit the exposure of patients, caregivers, and health care providers to COVID-19.
When the pandemic reached UT Southwestern, the response was immediate throughout the institution. COVID-19 provided a real-life laboratory to study modifications to long-standing clinical research practices. Simmons Cancer Center made decisions at the outset to do whatever we could to best support our workforce and make changes to our efforts and pace of enrollment as needed to keep our patients safe. We moved to remote informed consent, telehealth visits, performance of off-site diagnostics, delivery of oral medications to patient homes, and remote study monitoring, just to name a few changes.
Every position was assessed for remote work capabilities, equipment was deployed, and employees who could work from home were moved off campus. Direct patient-staff member interactions were minimized, and we took the time during our brief enrollment hold to write policies and procedures and train staff for the changes to clinical trials conduct we deemed necessary.
This approach ultimately enabled us to continue enrolling patients throughout three more surges of the virus.
Surveys Open a Window into COVID Impact
All of this was a learning experience like no other. In May 2020, we performed a baseline survey to explore the ways in which clinical research professionals perceived these changes to clinical research processes.
For our initial survey, we had an 87% response rate among staff members in our Clinical Research Office at Simmons Cancer Center, which employs more than 100 individuals. Here are the top things we found:
- Responses were slightly favorable for remote informed consent, performance of off-site diagnostics, and remote study monitoring.
- Enthusiasm was greater for telehealth visits and shipment of oral therapy to patients’ homes.
- Overall, the responses indicated a better perception of the new system than the old.
In a follow-up survey performed six months later, we found that research staff responded even more favorably to these changes.
We also asked respondents how they felt COVID-related adjustments affected various aspects of clinical research. While they felt that these adjustments had increasingly favorable effects on data quality and patient experience, over time they felt less favorably about effects on patient safety and their own experience. We infer that this trend might be due to the perception that remote, virtual assessments provide less effective patient monitoring or due to the respondents’ own pandemic fatigue, or both.
Overall, the surveys indicated considerable support for continuing pandemic-related changes to clinical trials in the future – many of which were long overdue.
Targeted Therapy in Kidney Cancer
On Aug. 13, 2021, the FDA approved the first-in-class drug belzutifan (Welireg), a hypoxia-inducible factor 2-alpha (HIF-2α) inhibitor, for adult patients with certain kidney cancers. This new kidney cancer treatment grew out of the discovery of the protein HIF-2α and its role in increasing oxygen supply during kidney cancer growth by Steven McKnight, Ph.D., Professor of Biochemistry, and David Russell, Ph.D., former Vice Provost and Dean of Research and Professor Emeritus of Molecular Genetics, at UT Southwestern in the 1990s.
Through the 2000s, the HIF-2α protein and its potential inhibitors were further studied by other researchers at UT Southwestern, including Richard Bruick, Ph.D., Professor of Biochemistry; Kevin Gardner, Ph.D., Professor of Biophysics; James Brugarolas, M.D., Ph.D., Director of the Kidney Cancer Program at Simmons Cancer Center and Professor of Internal Medicine; and Kevin Courtney, M.D., Ph.D., Associate Professor of Internal Medicine.
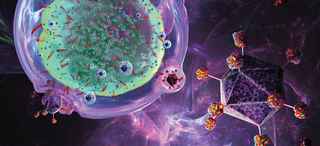
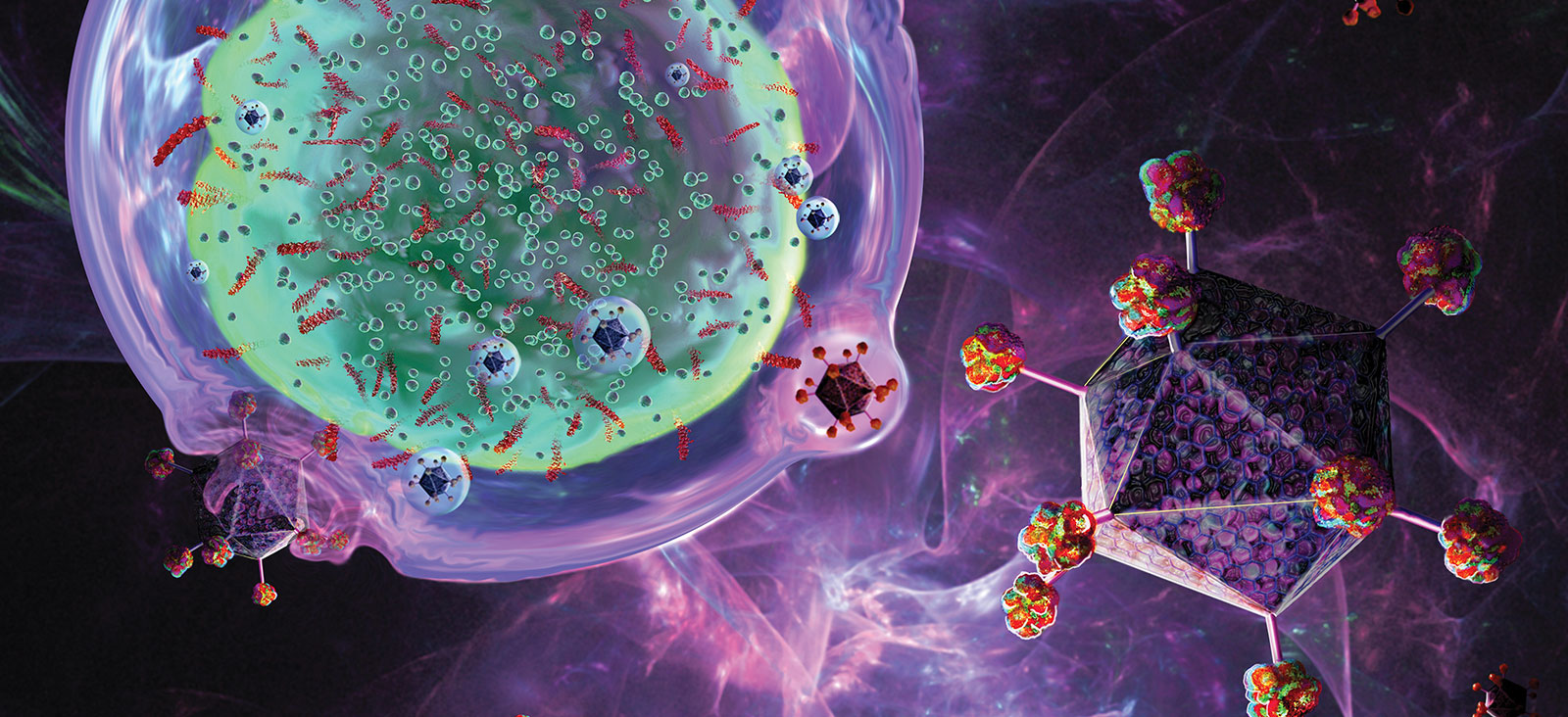
Updates on CAR T-Cell Therapy Clinical Trials in Adult and Pediatric Cancers
Simmons Cancer Center is a leader in developing and implementing chimeric antigen receptor (CAR) T-cell therapies that are improving outcomes for both adult and pediatric cancer patients. We are one of nine exclusive sites enrolling multiple myeloma patients in the KarMMa clinical trial. Samuel John, M.D., Assistant Professor of Pediatrics, has developed a promising CAR T-cell therapy for pediatric acute myeloid leukemia, which might move into clinical trials in the near future. Ankit Kansagra, M.D., Assistant Professor of Internal Medicine, is co-leader of the Global CAR T Initiative, a group of physicians who are drafting guidelines for using CAR T-cells.