A rare couple in the lab world has developed a new way for surgeons to look at the tumors they need to remove.
by Matt Goodman
Surgery isn’t as exact a science as we would like to think. Much is determined by the judgment calls of the man or woman who holds the scalpel — where to cut, what to cut, how to cut, when to cut. That can be especially tricky when it comes to surgically removing cancerous tumors. Cut too little, and there’s a chance that the cancer will continue to metastasize. Cut too much, and you put the organ at risk.
But what if there was a way to visually isolate tissue so a surgeon could cut precisely around a defined border, effectively taking the guesswork out of operating?
That was the question that Dr. Jinming Gao and Dr. Baran Sumer set out to answer when they teamed up 10 years ago at the University of Texas Southwestern Medical Center.

Earlier this year, a 130-pound Great Dane had cancer in the bone of his leg, one of the hardest types to operate on. The surgery was too tricky. So the Great Dane became the first patient to test the technology that came out of Sumer and Gao’s lab.
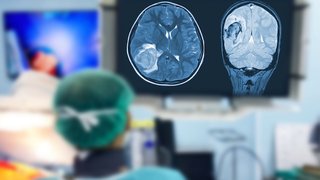
A nanosensor designed by Gao’s lab responds to the acidic pH that distinguishes tumor cells from the surrounding tissue. Then an unraveling of molecular dye causes the corresponding tissues to fluoresce. The surgeon now has a clearly defined tumor to cut out, lit up like a Lite-Brite. No more eyeballing.
The technology is being tested in a phase 1 human clinical study in cancer patients undergoing surgery including head and neck cancers at the University Medical Center in Groningen, Netherlands, under principal investigator surgeon professor Go van Dam.
If the technology is successful, Sumer and Gao believe surgeons everywhere will be able to remove cancerous tissue more accurately without harming any surrounding healthy tissue. It could mean cases that were previously inoperable now have a fighting chance.
If you think the dog was an unusual patient, Sumer and Gao are an unusual pair in the research and medical world.
If the technology is successful, Sumer and Gao believe surgeons everywhere will be able to remove cancerous tissue more accurately without harming any surrounding healthy tissue.
Gao is a scientist. Sumer is a surgeon. Mostly, Gao says, scientists and doctors “tend to stay siloed in their areas of expertise.” But Gao says it is necessary to put the two in the same room: Sumer provides insight into what would help a surgeon, while the Gao lab develops new technologies that solve Sumer’s needs in the operating room.
At UT Southwestern, it’s become idiosyncratic for biomedical scientists to work hand in glove with surgeons, as the Medical Center works to solve a larger question: How do you translate something from the lab to the operating room, from a study to an actual patient? And how do you do it quickly?
“People call this the Valley of Death,” Sumer says. “All these great ideas start in the basic science lab; thousands and thousands of ideas, and most of them — 99 percent, 99.9 percent — never reach the clinics.”
For Sumer and Gao, it’s not just about the discovery. Many scientists consider their work done when the paper is published, but the couple won’t be finished until they see their new technology in the hands of surgeons across the country.
OncoNano Medicine, a venture-backed startup cofounded by Sumer and Gao, who sit on the company’s scientific advisory board, is currently developing the technology and has secured grants from the Cancer Prevention & Research Institute of Texas and the National Institutes of Health. Sumer and Gao hope to see it in use in the next few years.
“Knowledge gain is good, but what’s the mission?” Sumer asks. “Research, discovery, yes, but also patient care.”
Image Source: ONM-100 Phase 1 (Shine) Study; University Medical Center Groningen, Tracer Center of Expertise, Netherlands