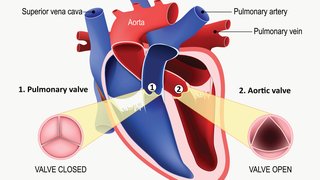
There’s a new class of drugs that dramatically lowers LDL cholesterol levels, and the story of this class of drugs begins at UT Southwestern Medical Center. The drugs are PCSK9-inhibitors, and a decade ago, researchers at UT Southwestern played a pivotal role in the identification of the protein PCSK9 as a target for lowering cholesterol.
In 1999, UT Southwestern physician Dr. Helen Hobbs launched the Dallas Heart Study, a multi-ethnic epidemiologic study of several thousand individuals in Dallas County. The study collected extensive health information about the participants, in conjunction with obtaining blood samples to determine genetic profiles.
In 2003, French scientists discovered the gene that codes for the PCSK9 protein and showed that a “gain-of-function” mutation in that gene caused very high levels of circulating LDL cholesterol, or bad cholesterol. At UT Southwestern, Dr. Hobbs and colleague Dr. Jonathan Cohen hypothesized that “inactivating” mutations might lead to lower levels of PCSK9 and low levels of circulating LDL cholesterol. They began looking for individuals with such PCSK9 mutations by combing through data from the Dallas Heart Study.
Markedly reduced incidence of heart disease
By focusing on Dallas Heart Study participants whose LDL cholesterol levels were at the lowest end of the range, the two researchers found two different mutations in the PCSK9 gene that resulted in loss of function. One of every 50 African-Americans in the study had one of these mutations, and indeed had low LDL cholesterol. Subsequently, they showed that individuals with inactivating mutations in PCSK9 have a markedly reduced incidence of heart disease.
The two geneticists’ search didn’t stop there. In 2006, Drs. Hobbs and Cohen announced that they had found a woman who had inherited not just one, but two mutations in the PCSK9 gene – one mutation from each parent. This woman, an aerobics instructor, had startlingly low levels of LDL cholesterol: Her LDL was 15 mg/dL, whereas anything below 100 mg/dL is considered optimal. Crucially, this woman suffered no ill effects from her extremely low LDL cholesterol, suggesting that therapies aimed at blocking or reducing PCSK9 would not only be effective but also safe.
Galvanizing industry efforts
This finding helped galvanize industry efforts to develop this new class of drugs that have the potential to change the course of discussion related to high cholesterol. The PCSK9-inhibitors developed by the drug companies are administered by monthly (or twice-monthly) injection. The FDA approved one of the new drugs, Praluent, in July 2015 and the second, Repatha, was approved in August 2015.
Praluent and Repatha will be prescribed for individuals with very high cholesterol that is not sufficiently controlled by statins.