Clarifying the Mechanics of Brain Metastasis in Breast Cancer
April 12, 2023
A new study reveals how breast cancer metastasizes in the brain and how metabolic diversity and plasticity dictate its spread.
Brain metastases in breast cancer develop after the spread of cells from the primary tumor to the brain through vasculature. While most tumor cells disseminated in the brain parenchyma perish, the surviving few may initiate synchronous metastases that are detected along with the primary tumor or differentially adapt and stay latent for years.
The survival dependencies of cancer cells that have differentially adapted to the brain parenchyma are not well characterized. Understanding these differences in cancer cells with a similar genomic profile is vital for the development of effective treatments for patients who present with synchronous or delayed metachronous metastases.
“Metachronous brain metastases occur in up to 50% of HER2+ breast cancer patients considered disease-free after a variable length of time following primary diagnosis and treatment,” says Srinivas Malladi, Ph.D., Assistant Professor of Pathology at UT Southwestern. “Few studies have identified the role of the brain microenvironment and nutrient availability in shaping disseminated tumor cell metabolism and growth.”
New research published in Cell Metabolism by Dr. Malladi and his colleagues suggests that metabolic diversity and plasticity within brain-tropic cells determine metastatic fitness. Using models of synchronous, latent residual, and metachronous brain metastatic disease, he and his team have uncovered distinct metabolic states associated with HER2+ breast cancer brain-tropic cells.
“These findings can be used to better understand clinical management of brain metastases and guide differential therapeutic target selection,” Dr. Malladi says.
“These findings may also have broader therapeutic applicability to other cancers with a propensity to disseminate to the brain.”
Srinivas Malladi, Ph.D.

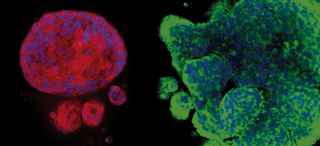
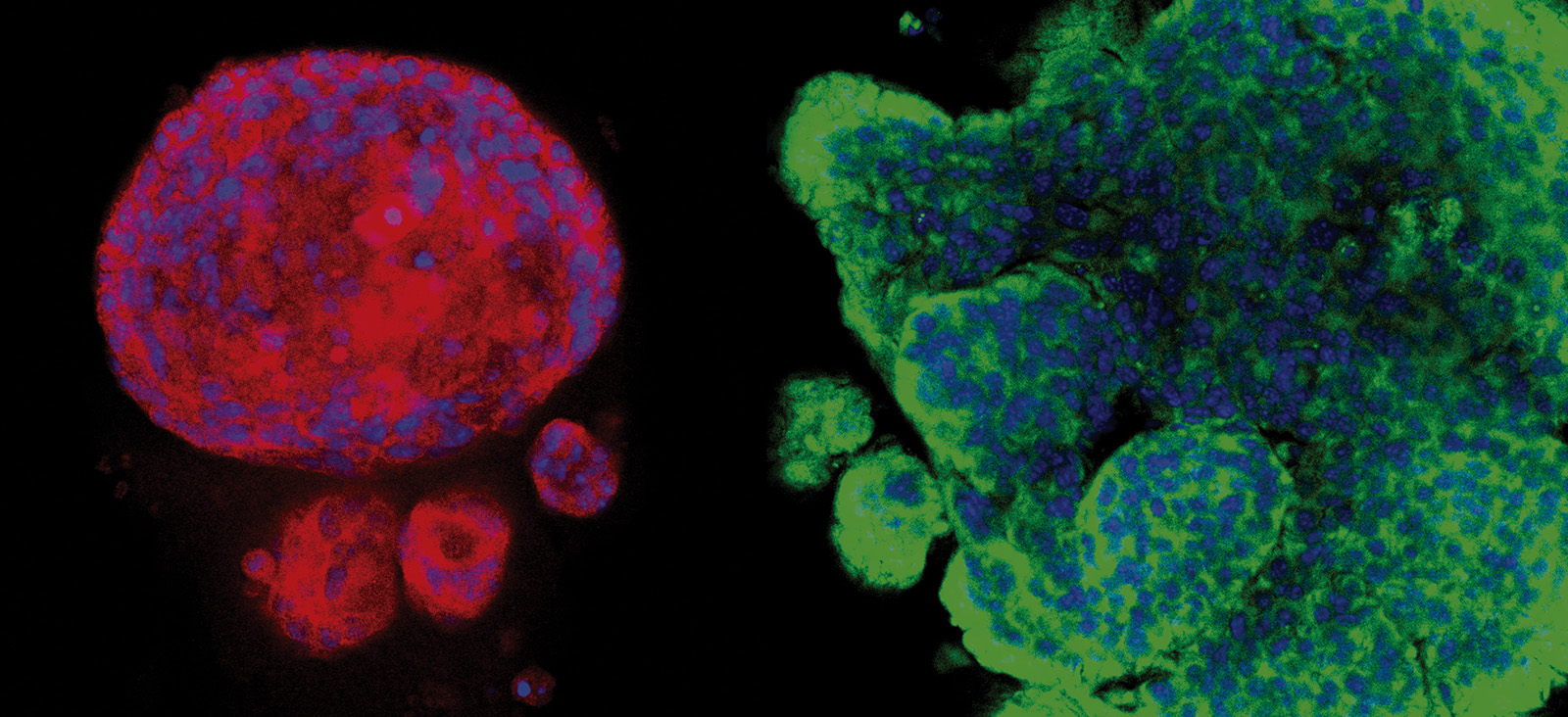
Brain Metastasis Models
Through a phenotypic screen in mice, the researchers isolated HER2+ synchronous (S-BM), latent residual (Lat), and metachronous (M-BM) brain metastatic cells. By investigating these phenotypically distinct brain-tropic S-BM, Lat, and M-BM cells, they discovered the impact of metabolic diversity and adaptations on metastatic fitness and identified metabolic vulnerabilities in these cell populations.
Mechanistically, they found that lactate secreted by aggressive metastatic cells or lactate supplementation to mice bearing Lat cells limited innate immunosurveillance and triggered overt metastasis. Additional findings indicated that attenuating lactate metabolism in S-BM impeded metastasis, while M-BM adapted and survived as residual disease.
“These data indicate that tumor cell-secreted lactate modulates NK cell cytotoxicity,” Dr. Malladi explains.
xCT expression
Other preclinical investigations revealed that in contrast to S-BM, Lat and M-BM survived in equilibrium with innate immunosurveillance, oxidized glutamine, and maintained cellular redox homeostasis through the anionic amino acid transporter xCT. Furthermore, Dr. Malladi and his co-investigators found that xCT expression was significantly higher in matched M-BM brain metastatic samples compared to primary tumors from HER2+ breast cancer patients.
“In our study, xCT-mediated cellular redox homeostasis promotes metastatic latency and relapse,” Dr. Malladi says. “Overall, inhibiting xCT function attenuated residual disease and recurrence.”
Therapeutic Implications
Current systemic therapies targeted toward HER2+ brain metastatic disease are not curative, and patients with S-BM, Lat, or M-BM brain metastases are treated with the same regimens despite differences in clinical presentation. Dr. Malladi further explains that these findings suggest several potential approaches could be leveraged to limit residual disease and outgrowth of brain metastasis.
“Pharmacological inhibition of xCT or glutamine metabolism in combination with current standard-of-care anti-HER2 drugs could be therapeutically beneficial to limit residual disease, potentially delaying metastatic relapse,” Dr. Malladi says. “The erastin analog PRLX 93936 is currently undergoing clinical testing in multiple myeloma, and clinical use or trials of this strategy to delay brain metastasis in HER2+ metastatic breast cancer patients are warranted.”
He adds: “These findings may also have broader therapeutic applicability to other cancers with a propensity to disseminate to the brain.”
Srinivas Malladi, Ph.D., is an Assistant Professor of Pathology at UT Southwestern and a member of the Cellular Networks in Cancer Program at Simmons Cancer Center. His research is focused on understanding how disseminated cancer cells survive and give rise to overt metastasis at a cellular and molecular level using a multidisciplinary and integrative approach.