In March 2019, media outlets hailed two new studies on transcatheter aortic valve replacement (TAVR) as potentially signaling the beginning of the end of open heart surgery for aortic stenosis. Data from these studies supported the U.S. Food and Drug Administration's approval of TAVR for low-risk individuals – replacing a narrowed aortic valve through a small incision in a procedure performed in the cardiac catheterization laboratory or hybrid operating room, as opposed to open heart surgery – for patients with low surgical risk. As many as 50 percent of patients with aortic stenosis are low-surgical risk patients.
After the presentation of those landmark studies and the FDA approval of TAVR for low-risk individuals, many predicted the beginning of the end of open heart surgery. Some were concerned about how open heart surgery is portrayed in the media, such as in this article in The New York Times that referred to “cracking open the ribs” and “stopping the heart.” While medically accurate, that type of verbiage is likely to be scary to patients and overly dramatic. However, two years have passed, and surgery still remains a viable and important treatment for aortic valve disease.
It’s understandable that patients are excited about TAVR, which offers immediate benefits of shorter recovery and less time spent in the hospital. Still, some some long-term unknowns remain surrounding TAVR for low surgical risk patients. Before we dive into that discussion, let’s go through the research on the topic.
Details about the studies
Two separate studies presented at the March 2019 American College of Cardiology conference supported similar findings: After one to two years of follow-up, TAVR outcomes were found to be similar to surgical aortic valve replacement (SAVR) outcomes in patients with aortic stenosis and low surgical risk.

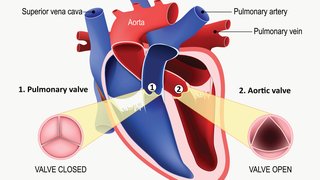
In a TAVR procedure, a doctor can repair a diseased aortic valve without open heart surgery. The new heart valve is inserted via catheter through an incision in the groin, guided inside the affected valve, and expanded to reopen it. Approximately half of patients with aortic stenosis today are considered to have low surgical risk – hence the excitement about those studies.
Medtronic’s trial studied the CoreValve platform. Researchers assessed two endpoints after two years: death and disabling stroke. This study found that 5.3 percent of patients who had TAVR died or suffered a disabling stroke compared to 6.7 percent of patients who had surgery. All-cause mortality rates were the same for both procedures. Disabling stroke affected 1.1 percent of TAVR patients and 3.5 percent of surgery patients. The mean age of participants was 74.
The Edwards Lifescience’s trial studied the SAPIEN 3 valve. The three trial endpoints were death, stroke, or re-hospitalization after one year. The data showed that outcomes with TAVR were significantly better than outcomes with surgery. After a year, deaths related to stroke or re-hospitalization related to the disease, valve, or procedure occurred in 8.5 percent of patients who had TAVR and 15.1 percent of patients who had surgery. The mean age of participants was 73.
The two competing medical device companies have both since published two-year results, which are in line with the initial reported findings.
While I think it’s reasonable to consider TAVR for the right patient population, low-risk patients should recognize the risk and uncertainty baked into that decision. Their TAVR procedure could be done well, giving them reliable 10- to 20-year outcomes. Or TAVR could benefit the patient only in the short-term (shorter hospital stay, shorter recovery), but the patient might need additional surgery in the future.
Dharam Kumbhani, M.D.
Medical Mysteries
Her heart was failing only a few years after a valve replacement, but it wasn't clear why. Examine the scan, put on your medical detective hat and see if you can form the right diagnosis. Dr. Kumbhani will provide the answer in the video at the bottom of this article.
3 concerns about the TAVR studies
1. The data were relatively short-term, and patients might need surgery later.
The studies followed patients for only one to two years. Data showed that these patients had similar or better outcomes compared to surgery in the midterm. However, aortic stenosis is a degenerative disorder, so it is more common in older patients. And because TAVR candidates have traditionally been a sicker cohort, we do not have substantial data on the effectiveness of TAVR beyond five years or so after placement.
Medical literature suggests the lifespan of the tissue valves used in surgical aortic valve replacement is typically about 10 to 20 years. Since TAVR valves are made of the same biological tissue, it is expected they will have the same longevity. However, we don’t have data to verify that. After the valve wears out, a patient will need to have a new valve placed or have another valve implanted during open heart surgery.
A 65-year-old patient likely could be expected to live longer than 10 to 20 years after surgery, so durability of tissue valves must be validated. Valve replacement or revision might be simple if the patient can have another TAVR procedure – a new tissue valve can be placed inside the previous valve. But if the patient needs surgery, the doctor would have to remove the existing valves and then replace them. Unfortunately, most surgeons do not have a lot of experience in removing CoreValve and SAPIEN 3 valves yet, which could pose a risk to patients.
2. TAVR in low-risk patients is FDA-approved, and many people want it now.
When patients with low surgical risk call and want to switch to TAVR, we engage them in shared decision making. It is important to keep in mind that patients with bicuspid valves also were excluded from these trials. Patients with bicuspid valves develop aortic stenosis earlier in life and may frequently be low-risk patients.
While I think it’s reasonable to consider TAVR for the right patient population, low-risk patients should recognize the risk and uncertainty baked into that decision. Their TAVR procedure could be done well, giving them reliable 10- to 20-year outcomes. Or TAVR could benefit the patient only in the short-term (shorter hospital stay, shorter recovery), but the patient might need additional surgery in the future.
Comprehensive cardiovascular care at UT Southwestern

Valve Disorders
Our doctors are experts on innovative treatments, including minimally invasive surgery, that can help patients recover more quickly and feel better faster.

Coronary Artery Disease
Our heart specialists use the latest scientific insights and advanced therapies to help people with or at risk for coronary artery disease prolong and improve the quality of their lives.

Find a Clinical Trial
Search for opportunities to participate in a heart or vascular research study.
Also, it is important to realize that patients who were eligible for mechanical valves were excluded for these trials. Typically, patients younger than 50 are offered mechanical valves. Mechanical valves are designed to last for the rest of a patient’s life. While the durability of mechanical aortic valves is excellent, patients must take a blood thinner (warfarin) to maintain valve function.
3. The benefits of TAVR may not yet balance potential long-term risks.
Patients who get TAVR likely will have a shorter recovery. There are no bones and muscles to heal – just some tenderness in the groin incision.
However, is the risk of needing another TAVR procedure or a potentially complex surgery down the line preferable to these benefits? Some patients might say yes. But as a doctor, I say we need more research to determine the safest route – especially because we know surgery provides good outcomes for low-risk patients today.
Another important consideration is a risk of forming clots on the tissue valves. First described for TAVR valves in 2015, it is frequently asymptomatic and requires a CT scan for a definitive diagnosis. We don’t know if clots are more frequent with TAVR valves compared with surgical valves, but current literature suggests that about 1 in 10-15 patients undergoing TAVR might develop this condition. In fact, as part of the trial design, it is required that approximately 300 patients in both the low-risk trials undergo follow-up CT scans to accurately assess the incidence of this problem and determine whether it is different between TAVR and surgery. The long-term significance of these clots and the best way to treat them also is not completely understood.
The future of TAVR
The landscape is going to be very interesting over the next few years. I imagine that valve manufacturers will do a lot of direct-to-consumer advertisements to sway people against open heart surgery in favor of TAVR. And for some patients, that might be a good option. We’ll be able to say that more confidently once we have long-term data on the safety and durability of TAVR valves.
I empathize with patients who want minimally invasive options. It has to be frustrating to be told that surgery is the only choice today. But know that the heart team at UT Southwestern’s Clinical Heart Center has our patients’ best interests in mind. We want to give our patients more healthy years to enjoy the people and activities they love.
Above all, we want our patients to get the right treatment that will give them the best results for their unique condition. And the best way to do that is to gather long-term data and help patients make informed decisions about their care.
If you or a loved one would like to discuss treatment options for aortic stenosis, call 214-645-8300 or request an appointment online.
Medical Mystery: Revealed
Dr. Kumbhani and his colleagues across the country said this was one of the most challenging cases they had seen in recent memory. But they followed the clues and ultimately saved the patient. Did you solve the case, too?